|
I guess I should have used scare quotes. It was a metaphor. Models taught to high schoolers, at least where I went to school, start with abstractions like 2,8,8,2 and by the final year of school is just starting to talk about orbitals, spin and energy levels. 1st year of university starts to talk about the shape of these orbitals, again abstracted. You can't try to teach simplified versions of 1st year chem to 13 year olds, you need something they can understand and the progression as they advance mirrors in a lot of ways the progression of understanding of these concepts in the past. Tl;dr: it's not wrong, just incomplete.
|
|
|
|

|
| # ? Apr 23, 2024 22:59 |
|
How to do use energy to make atoms gain/lose electrons to make a lighter element become a heavier one? Like, how to do gently caress around with the Uranium that comes out of the ground in one form and make it go from the Virgin Uranium to the Chad Uranium-235 that you can use in nuclear reactors? If Uranium has a specific half-life, what makes it become Cool Uranium that has the correct density to power powerplants and thus have a different half-life? I understand "half-life" has a concept, and radioactive decay - but how does uranium hang around long enough for us to make it into Cool Uranium that makes power then decays into a whole bunch of other bullshit. How do you blow up a nuke using Plutonium and have the products be, for example, cesium-137? As you can tell I simultaneously have an excellent, and ZERO understanding of nuclear physics. If you pumped enough energy into a Hydrogen atom, could you force it to be literally any other atom, so long as you had the energy available to add electrons? How do you make an atom have more protons and neutrons than it had to begin with? Also, AAAAAAAAAAAAAAAAAAAAAAAAAAAAAAA I've always struggled immensely with math, chemistry and physics, but many years of Youtube nerds have helped me... however I feel like I'm still missing the basics.
|
|
|
|
Caedus posted:How to do use energy to make atoms gain/lose electrons to make a lighter element become a heavier one? Like, how to do gently caress around with the Uranium that comes out of the ground in one form and make it go from the Virgin Uranium to the Chad Uranium-235 that you can use in nuclear reactors? If Uranium has a specific half-life, what makes it become Cool Uranium that has the correct density to power powerplants and thus have a different half-life? I think you're confused what an electron is.
|
|
|
|
Caedus posted:How to do use energy to make atoms gain/lose electrons to make a lighter element become a heavier one? Like, how to do gently caress around with the Uranium that comes out of the ground in one form and make it go from the Virgin Uranium to the Chad Uranium-235 that you can use in nuclear reactors? If Uranium has a specific half-life, what makes it become Cool Uranium that has the correct density to power powerplants and thus have a different half-life? Itís all leverage and gear ratios and then hydraulic forces when you start talking about outer valences. Quantities of power have practically gently caress all to do with chemistry or electrons. It only really comes into play in alchemy when you are trying to change the gauge or scope of the proton. All electrons are like, full power, all the time. ClamdestineBoyster fucked around with this message at 06:45 on Apr 3, 2021 |
|
|
|
I think maybe you're thinking of this: https://en.wikipedia.org/wiki/Electron_capture ? An atom's element is determined entirely by its proton count. If you have 8 protons and 8 neutrons and 8 electrons, it's an oxygen atom. If it has 8 protons, 8 neutrons, and 30 billion electrons - it's still an oxygen atom. Indeed, if it has has 8 protons, 889897 neutrons, and 0 electrons, that's also an oxygen atom. Nuclear physics is generally concerned mostly with the nucleus (hence the name), i.e. protons and neutrons. Uranium-235 is named for the number of protons + neutrons it has. Same with Uranium-238 or anything else. The electrons are just assumed to be there or not matter much. If you want something to have more protons or neutrons, you need to throw protons or neutrons at it and hope they stick. (The process is slightly complicated by the fact that if it's unstable, a proton might turn into a neutron or vice-versa, but that's the basic idea.) I have oxygen (8 p and 8 n) and want aluminum (13 p and 13 n), I could blast it with boron (5 p and 5 n). 8 + 5 = 13. If you want it to have fewer, you shoot it until it breaks apart, or becomes unstable and falls apart on its own. So, if we want plutonium from uranium, we can do this: https://wikimedia.org/api/rest_v1/media/math/render/svg/f9ba9e4744226a97ce8a41fd5b5e50b18cc259a9 We take Uranium-235 and add 1 neutron. This isn't stable, so the neutron becomes a proton. The atom now has 93 protons, and it becomes Neptunium. This also isn't stable, so another neutron becomes a proton, and it become Plutonium-236. You can see 235 + 1 = 236. It went through beta decay twice, which means two electrons flew off the nucleus, yet it went UP on the periodic table. Forcing electrons into the atom, would technically bring it closer to hydrogen, not away from it. You're kind of on the right track about the part where you asked about hydrogen atoms. Every atom in the universe is built up from hydrogen atoms in stars and supernovae (and maybe neutron star collisions). Not all half-lives are so short. Bi-209 undergoes alpha decay with a half-life of approximately 19 exayears (1.9◊10^19, approximately 19 quintillion years), so you have plenty of time to mine it up. Maybe someone else can give a better explanation, but that's the gist. Shadow0 fucked around with this message at 07:03 on Apr 3, 2021 |
|
|
|
All atoms have one proton, the heft varies with the relative draw of the electron valances.
|
|
|
|
ClamdestineBoyster posted:All atoms have one proton, the heft varies with the relative draw of the electron valances. There's only one electron in the entire universe, and it travels through time and space at near instantaneous speeds. Positrons are just whenever it misses a stop and has to back up.
|
|
|
|
Quantum.
|
|
|
|
is there any real rule to using house cleaners other than avoiding bleach + ammonia i use simple green for everything 
|
|
|
|
How does the machine cut what is essentially a waveform into a record, how does a needle play back these bumps and grooves as that song. I've read up on it but it just doesn't make sense to me.
|
|
|
|
This thread is terrific. If complex physics and chemistry qualify as "basic poo poo" we don't understand, I'm a little intimidated. How does adhesive tape work? Or rather, how does adhesive tape deteriorate? Give it a year or two, and it seems everything from Scotch tape to packing tape to duct tape turns into a dried, flaky, fibrous mess. I'm guessing it's something to do with water? Once the last of it has evaporated, I'm just left with dried gunk that has no holding power?
|
|
|
|
Shadow0 posted:I think maybe you're thinking of this: https://en.wikipedia.org/wiki/Electron_capture ? I definitely think my brain is fusing two different physics phenomena into one. I told you I had an excellent/poor understanding of this! This is what I mean, despite the hours of cute physics youtube I watched I've clearly missed something! I heard about elements that donate elections and something about ions which I don't get either. If donating an electron is completely different from an atom gaining or losing a proton or neutron, how does that happen? I get that the elements higher on the table are less stable on the lower one, which is why there's so much hydrogen floating around and not much Americium, but I don't understand the mechanism of yelling at Uranium enough until it becomes Plutonium that you can build a nuke with. You just like.. shoot the Uranium with another atom that will give it the extra proton/neutron to become Plutonium? Does the process work in reverse, to make a lighter element? I feel like I'm in the same spot I am with Biology wherein I understand DNA and how it replicates on a basic level but also.. how the gently caress can it do that! I get the parts but not how they go together.
|
|
|
|
Why and what the gently caress do women carry so much poo poo all the time? Okay so I work at a university right and I see lots of professional women. Some of them carry 3, yes THREE bags. A huge hand bag, a backpack and another carry bag. WTF is in there??
|
|
|
|
women be shopping
|
|
|
|
Sometimes people go through their lives carrying a lot of baggage.
|
|
|
|
Connormgs posted:How does the machine cut what is essentially a waveform into a record, how does a needle play back these bumps and grooves as that song. I've read up on it but it just doesn't make sense to me. I know how it was done with phonographs that used wax cylinders, I'm assuming the tech for records is a more advanced version of the same thing. With wax cylinders, you heat the wax until it's soft, not melted, and you play sound into the phonograph, causing the needle to bounce and tremble, cutting and molding the wax into the waveform of the sound.
|
|
|
Suspicious Lump posted:Why and what the gently caress do women carry so much poo poo all the time? Okay so I work at a university right and I see lots of professional women. Some of them carry 3, yes THREE bags. A huge hand bag, a backpack and another carry bag. WTF is in there?? Day-to-day stuff, Laptop plus papers, and maybe gym clothes or something?
|
|
|
|
|
ikanreed posted:I know how it was done with phonographs that used wax cylinders, I'm assuming the tech for records is a more advanced version of the same thing. Disc records are easier to make than wax cyinders, which is why everybody ditched cylinders fast. Since all the recording grooves are in a single plane, you can just use a lathe to cut a metal master, then create a negative of the master and use it to mass produce pressed records. Also IIRC the original wax cylinders produced sound by varying the groove depth while modern records use horizontally wobbly grooves for the same effect, which is apparently also easier to reproduce. steinrokkan fucked around with this message at 16:35 on Apr 3, 2021 |
|
|
|
It seems weird that how wobbly the groove is generates multiple instruments
|
|
|
|
|
Connormgs posted:How does the machine cut what is essentially a waveform into a record, how does a needle play back these bumps and grooves as that song. I've read up on it but it just doesn't make sense to me. Essentially the machine acts like your ears. Sound goes in, hits the drum to produce vibrations, and those vibrations are then translated to a cutting needle on a soft vinyl record that makes the physical sound wave that was produced.
|
|
|
|
Caedus posted:I definitely think my brain is fusing two different physics phenomena into one. I told you I had an excellent/poor understanding of this! This is what I mean, despite the hours of cute physics youtube I watched I've clearly missed something! I heard about elements that donate elections and something about ions which I don't get either. An unstable nucleus can decay by a few mechanisms, some of which involve throwing out a neutron or occasionally an entire helium, resulting in a lighter element. If you smash a suitable nucleus hard enough then it can also just break into a couple of smaller nuclei. That's fission. That's probably basic enough.
|
|
|
|
Goodpancakes posted:It seems weird that how wobbly the groove is generates multiple instruments Well, that's real tricky and gets into how ears work. You have little hairs of different lengths inside your ear, each one basically representing a narrow frequency range that can both reach their chamber and cause them to harmonically oscillate. Mathematically, the results are incredibly similar to a fourier transform. If they oscillate, they activate their attached neuron. Your brain then basically does pattern recognition for what collection of harmonics correspond to what "voices" So if a waveform harmonically oscillates on a certain wavelength, regardless of whether that's stacked with other waveforms your brain can kind of pick it out. Unless that combined stack happens to hit a different pattern of activation. Then you might get a yanny/laurel situation.
|
|
|
|
Connormgs posted:How does the machine cut what is essentially a waveform into a record, how does a needle play back these bumps and grooves as that song. I've read up on it but it just doesn't make sense to me. Sound is a wave, so essentially you cut a similar wave into the record. As the needle moves along the groove, it will move along the wave. Then there's a magnet and a coil. As the needle moves, the magnet and coil move relative to each other, generating an electric wave. Then you boost that signal, and have another coil and magnet, this time the other way around. The coil pushes the magnet, which pushes a drum, which pushes air. So you turn a physical wave into an electric wave and then back into a physical wave using magnets. Edit: To make the records, just do it in reverse. Sound comes in, drives a magnet, which moves a knife or whatever to cut the ridges. Shadow0 fucked around with this message at 18:28 on Apr 3, 2021 |
|
|
|
Caedus posted:I definitely think my brain is fusing two different physics phenomena into one. I told you I had an excellent/poor understanding of this! This is what I mean, despite the hours of cute physics youtube I watched I've clearly missed something! I heard about elements that donate elections and something about ions which I don't get either. There's less Americium because Americium is made from hydrogen at extremely, extremely high levels of effort. Much like how there are more trees than mansions in the world. Or how many grains of sand there are in the world vs wine glasses. In the beginning, there was only hydrogen. Then some other elements got made when stars exploded. How many exploding stars are around you right now? Not toooo many. The largest stars in the universe can only make up to iron before dying. It's only in the extreme processes of supernovae that even a tiny amount of heavy stuff can be made. You can get more protons by adding a proton or by adding a neutron that decays into a proton. You can get more neutrons by adding a neutron or by adding a proton that decays into a neutron. That's it. Electrons aren't important except in the process of turning a proton into a neutron or vice-versa, since the charge needs to be preserved (protons are positive, and neutrons are neutral, and electrons are negative). Yes, that's right. So for a bigger element, it looks like this (typically called "fusion"): For a smaller element, it looks like this (typically called "fission"):  Notice: no electrons involved at all (or at least they aren't important enough to show). You might like this chart: 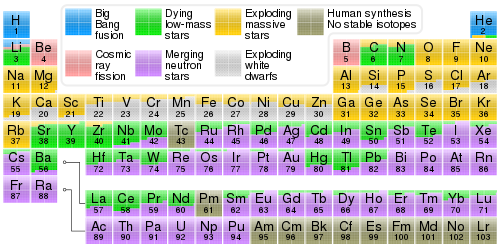 There's also this one: https://qph.fs.quoracdn.net/main-qimg-ba459074e47537689dff7875d076603c.webp We start with hydrogen on the left and gradually build up to iron using only more and more hydrogen. Shadow0 fucked around with this message at 18:39 on Apr 3, 2021 |
|
|
|
In some sense, electrons come and go all the time. Copper is still copper if you mess with the electron counts, or if it's in a molecule with some other element that lends or borrows some electrons from it. Very roughly speaking, of course - "lending electrons" is on the long "lies told to children" list. But it's a useful model. Different amounts of neutrons give you different isotopes. Some are entirely stable, some decay after a while (microseconds to millions of years, depending). Some neutron amounts are so practically impossible that the nucleus breaks apart before you get there. Uranium is element 92, which means it has 92 protons. When you dig it out of the ground, most (about 99.3%) is U-238 , which means it has 238 protons+neutrons (92 P + 146 N). Most of the rest is U-235 (92P + 141N). As talked about above, U-238 plus a neutron decays through Neptunium to Plutonium-239, apparently over a few days on average. U-235 plus a neutron typically results in the nucleus fissioning into Krypton and Barium - and three new Neutrons. What that means is that if you have a lump of U-235 and fire a neutron at it, that will near-instantly create three new neutrons that can then hit more U-235 and create more neutrons and boom. The "critical mass" of U-235 is the mass where, on average, the neutrons from each fission set off one new fission. If the average goes higher, it accelerates, if it goes lower it peters out. Obviously, this depends on the shape (a ball is more efficient than a plate or stick), but also on the surroundings; some materials reflect neutrons. Nuclear weapons work better with really high U-235 concentrations - you want the steepest acceleration possible. Reactors are fine with lower concentrations, you want to stay just around the critical point. Plutonium-239 works much like U-235 - they are both fissile (capable of sustaining a chain reaction). This also means that the U-238 in reactor fuel is useful: It turns into Pu-239 which also contributes to the chain reaction and heat output. The 99.3 : 0.7 ratio is what's left from the output of the supernova explosion(s) that created the building blocks of the solar system. U-235 has a shorter halflife, so as time passes there is more 238 and less 235 left. To separate the two, we use practical, physical means. The 238 atoms are a tiny bit heavier, so if you shoot a beam of charged atoms, and bend it a bit, the lighter 235 will bend just a bit more. Or you can make some sort of uranium liquid or gas, and then centrifuge it: The heavier 238 will sink to the outside of the tube. It's very far from perfect, but if you repeat it enough times you can slowly increase the concentration - you enrich the Uranium. This is what the Iran nuclear deal is about : They have these centrifuges, and they claim they are using them to nudge the 235 concentration up just enough to use for reactor fuel. This is also what the Stuxnet virus was for: It specifically targeted the control computers for those centrifuges. (And then it got onto the general internet). The opposite of enriched uranium is depleted uranium - basically pure U-238. This is still mildly radioactive, and also poisonous in the same way as lead - but it's kind of borderline safe. We make anti-tank bullets and tiny-but-heavy counterweights for aircraft out of it. Plutonium is easier: Put a Uranium fuel rod into a reactor and let it bake, then pull it out after a while; some of the U-238 will have turned into Pu-239. They are different elements, with different solubilities and chemical properties, so you can do more normal chemistry to separate them. For someone like Iran, what keeps them from doing this is more that it's kind of obvious - used fuel rods are insanely death ray-style radioactive, so you need a very specialized plant to work with them. The usual procedure is just to let them sit in water for years to quiet down, and then eventually you think about where to bury them. Enrichment plants have a legitimate use, but processing used fuel rods screams "I'm building nuclear weapons". (You could reprocess them into new reactor fuel, but that's a whole debate. The US doesn't like it when you do, since it's so easy to redirect some Plutonium to a weapons program.) Computer viking fucked around with this message at 22:41 on Apr 3, 2021 |
|
|
|
Computer viking posted:In some sense, electrons come and go all the time. Copper is still copper if you mess with the electron counts, or if it's in a molecule with some other element that lends or borrows some electrons from it. So itís either dry or saturated.
|
|
|
|
bigperm posted:My Grandma was super into spinning. She did it at (18th Century) reenactments all the time. To turn fibers into thread you have to 'card' it first. That gets all the fibers going in the same direction. When it's ready to spin, you literally are just twisting the fibers around each other a lot, and since they are all going in the same direction, they get caught up with each other and end up with a tensile strength much greater than a ball of wool. In general twist is the key to how it works. Like if you pull a regular cotton ball apart, you can do it super easily. But if you twist it into a tight cylinder, it's a lot harder to pull it apart, because the twist gives every little fiber so much more friction.
|
|
|
|
Kazzah posted:I know nothing about dog breeds. Like I understand the concept and I can recognise four or five, but other than that I don't know what any of their names are. I'll be in a conversation and someone will go "oh he's a northern jack peregrine spaniel cross", and everyone nods knowingly. It's like living in a world where everyone just knows all about fencing and what all the sword moves are called. I love that the op was instantly ignored and everyone just started answering questions
|
|
|
|
honestly, same
|
|
|
|

|
| # ? Apr 23, 2024 22:59 |
|
Knot My President! posted:is there any real rule to using house cleaners other than avoiding bleach + ammonia Basically bleach is chlorine based and alkalai. If you mix, for instance, vinegar with bleach you get chlorine gas. Mixing it with ammonia makes chloramine gas. Both are bad for you. Bleach and alcohol makes chloroform. This is also not good for you. Notice all that "chlor" repeating in these combinations? That's from the bleach. Don't mix cleaning chemicals.
|
|
|






















 i like nice words
i like nice words




